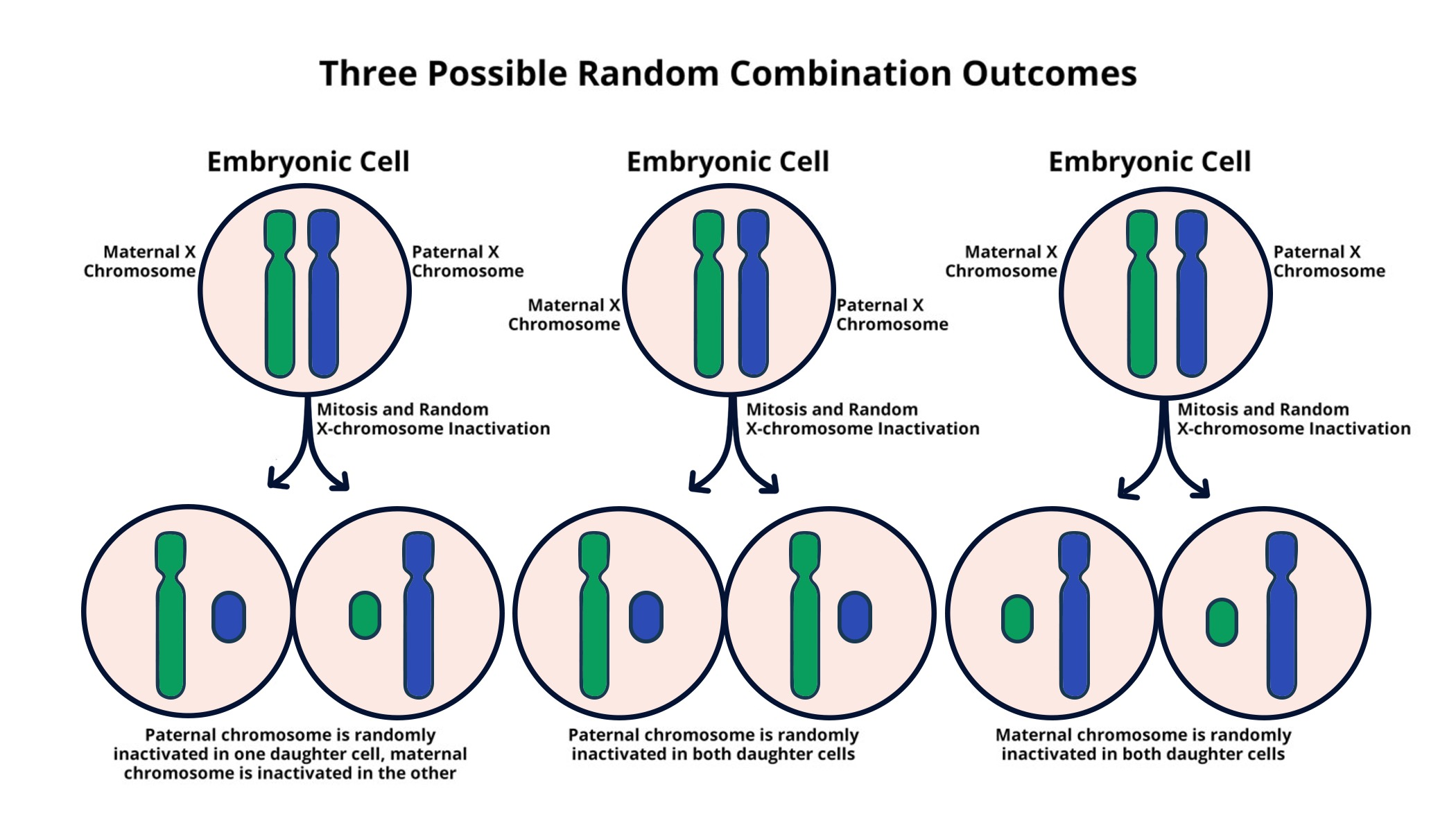
The phenomenon of X chromosome inactivation is a fascinating aspect of genetics that plays a crucial role in balancing gene expression between sexes. In females, who possess two X chromosomes, one is randomly silenced to prevent an overload of gene products, a process essential for healthy development. This chromosomal silencing, spearheaded by researchers like Jeannie T. Lee at Harvard Medical School, holds significant implications for the treatment of genetic disorders such as Fragile X Syndrome and Rett Syndrome. Understanding how this inactivation occurs could lead to pioneering therapies that potentially unsilence beneficial genes, offering hope to those affected by these conditions. As scientists delve deeper into the mechanics of this process, the prospect of innovative genetic disorders therapy becomes increasingly attainable.
X chromosome inactivation, often referred to as X-inactivation, is a critical genetic mechanism where one of the two X chromosomes in females is rendered inactive to balance gene dosage with males. This chromosomal repression is a key focus of contemporary scientific studies and is particularly relevant in the context of disorders linked to X chromosome abnormalities. Jeannie T. Lee’s groundbreaking research at Harvard has shed light on the intricate processes involved in this silencing, revealing potential pathways for developing effective treatments for conditions like Fragile X Syndrome and Rett Syndrome. By exploring the dynamics of this genetic regulatory system, researchers aim to create therapeutic strategies that exploit X-inactivation mechanisms, thereby addressing various genetic disorders and improving patient outcomes.
Understanding X Chromosome Inactivation in Depth
X chromosome inactivation (XCI) is a crucial biological phenomenon that ensures females, possessing two X chromosomes, do not express double the amount of X-linked genes as males with just one. This process is an elegant solution to gene dosage imbalance, achieved through silencing one of the X chromosomes in each female cell. Jeannie T. Lee’s groundbreaking research has been pivotal in unraveling the complex mechanisms underpinning XCI. By unveiling how Xist, an RNA molecule, interacts with surrounding chromosomal material, her team has illuminated pathways that can be leveraged for therapeutic innovations.
The implications of understanding X chromosome inactivation extend beyond basic science; they hold significant promise for developing treatments for X-linked genetic disorders. By focusing on how silencing mechanisms can be manipulated, researchers may restore expression to otherwise inactive genes that carry mutations responsible for conditions like Fragile X Syndrome and Rett Syndrome. This is particularly relevant as such disorders predominantly affect females due to their genetic backgrounds, but they also present challenges in male patients where similar silent mutations exist.
The Role of Chromosomal Silencing in Genetic Disorders
Chromosomal silencing is an intricate biological process whereby certain genes are turned off to maintain cellular homeostasis. In the context of genetic disorders, this mechanism plays a pivotal role in conditions linked to mutations on the X chromosome. For instance, mutations responsible for Fragile X Syndrome may be muted by the chromosomal architecture that Lee’s research describes, suggesting that understanding this silencing could lead to breakthroughs in therapy. The research underscores the need for a comprehensive grasp of how these silencing processes interact with gene expression.
Moreover, focusing on chromosomal silencing not only provides insights into treating conditions like Fragile X Syndrome and Rett Syndrome but also opens avenues for broader applications in genetic disorders therapy. Lee’s innovative approaches, which aim to selectively unsilence X-linked mutations, have the potential to unlock healthy gene expression from inactivated X chromosomes. With further research and development, we may soon have strategies that could alleviate symptoms and improve the quality of life for individuals affected by these challenging disorders.
Jeannie T. Lee’s Contributions to Fragile X Syndrome Research
Jeannie T. Lee, a leading figure in genetic research at Harvard, has made substantial contributions to understanding the interplay between chromosomal biology and genetic disorders, particularly Fragile X Syndrome. Her pioneering studies have delineated the mechanisms of X chromosome inactivation, providing crucial insights that could drive new treatment paradigms for this condition. With collaborations across multiple institutions, the findings aim to bridge basic science with clinical applications, transforming the understanding of such syndromes.
The implications of Lee’s work resonate in ongoing efforts to develop therapies for Fragile X Syndrome, as her lab’s research suggests that unsilencing the X chromosome could restore the function of disrupted genes. As the research progresses towards human clinical trials, the medical community grows increasingly hopeful about the potential for effective treatments that stem from these insights. The collaboration between laboratory discoveries and clinical application exemplifies the translational research model and the critical need for targeted intervention in genetic disorders.
Rett Syndrome: A Focused Area of Research
Rett Syndrome is a severe neurodevelopmental disorder predominantly affecting females and is characterized by a regression of developmental milestones. Jeannie T. Lee’s research offers hope for those affected by this condition, given its foundation in X chromosome biology. The unraveling of XCI mechanisms not only enhances understanding of Rett Syndrome pathology but also presents potential therapeutic strategies. By targeting the molecular processes involved in chromosomal silencing, Lee’s lab aspires to create methodologies that could reactivate the necessary genes for improved neurological function.
Moreover, advances in understanding Rett Syndrome through the lens of X chromosome inactivation reflect broader trends within genetics. With significant investment in research, there is an increasing emphasis on finding tailored treatments for disorders with an X-linked genetic basis. The potential for therapeutic strategies to target and unmask silent genes in Rett Syndrome is particularly exciting, as it suggests a revolutionary approach to treating disorders that have long lacked effective management options.
The Future of Genetic Disorders Therapy
The prospect of refining gene therapy strategies for genetic disorders, particularly those linked to X chromosome mutations, signals a new era in medical science. Jeannie T. Lee and her colleagues have laid a promising foundation for these advancements, with empirical research revealing methods to manipulate X chromosome behavior in various cell types. As work continues to hone these strategies, a future may emerge where disorders like Fragile X Syndrome and Rett Syndrome can be more effectively addressed through targeted interventions.
Future therapies might not only provide symptomatic relief but also potentially reverse symptoms by restoring gene function. As the research evolves, the hope is to validate these findings in clinical settings, paving the way for breakthrough therapies that utilize insights from chromosomal silencing principles established by researchers like Lee. The focus on XCI and its implications for wide-ranging genetic disorders therapy may redefine how clinicians approach treatment options and improve patient outcomes in the long run.
Xist and Its Implications in Chromosome Biology
The discovery of Xist as a key player in X chromosome inactivation has far-reaching implications in chromosome biology and genetics. Xist’s ability to coat the X chromosome not only facilitates its silencing but also illustrates the intricate relationship between RNA molecules and chromatin structure. Jeannie T. Lee’s research highlights how manipulating this interaction could enable scientists to develop therapies that target not just X-linked disorders, but also potentially alter gene expression patterns in other genetic diseases.
Understanding Xist’s molecular interactions opens doors to new strategies for gene therapy. As we deepen our insights into XCI, it becomes feasible to leverage these processes therapeutically, particularly for conditions like Fragile X Syndrome. By fine-tuning the mechanisms of how Xist silences genes, researchers may discover novel ways to reactivate benign genes, ultimately leading to therapeutic solutions that remedy genetic disorders at their source.
The Jell-O Analogy: Insights into Chromosomal Behavior
The analogy of the gelatinous ‘Jell-O’ that encapsulates chromosomes provides an intriguing perspective on chromosomal behavior and binding dynamics. This visualization, as proposed by Jeannie T. Lee, helps demystify concepts of chromosomal silencing and inactivation. By recognizing that cells utilize this viscous medium to separate and compartmentalize genetic material, researchers can better understand how specific regulatory elements operate to silence or activate genes.
Such an analogy also aids in comprehending the challenges associated with manipulating these chromosomal structures for therapeutic gain. As scientists explore how to unsilence X-linked genes for conditions such as Fragile X Syndrome, the characteristics of this ‘Jell-O’ become critical for designing effective interventions. This profound understanding may lead to scalable solutions that transform the way we approach treatments for various genetic disorders linked to the X chromosome.
Research Funding and Its Role in Advancing Genetic Studies
The financial backing from entities like the National Institutes of Health has been instrumental in advancing genetic research, particularly in the field of chromosomal inactivation. Jeannie T. Lee’s studies epitomize how prolonged funding can yield significant breakthroughs in understanding complex biological questions about X chromosome dynamics. Such funding allows researchers to pursue innovative ideas that may not have immediate commercial applications but are critical for addressing fundamental challenges in medicine.
The support for Lee’s work underscores the importance of long-term investments in basic scientific research, as these funding streams enable the exploration of novel therapeutic avenues for genetic disorders. As researchers continue to unveil the complexities of X chromosome inactivation, sustained funding will be crucial to bridge the gap between basic discoveries and clinical applications, ultimately benefiting countless individuals affected by genetic disorders.
Clinical Trials: Translating Research into Treatments
The journey from lab-based research to clinical trials is a pivotal phase in the development of new treatments for genetic disorders. Jeannie T. Lee’s lab is gearing up for this transition with promising findings on unsilencing X-linked genes associated with disorders like Fragile X Syndrome and Rett Syndrome. As the research progresses toward safety studies and clinical trials, it exemplifies the bridge between basic research and therapeutic application, signaling hope for innovative treatment options.
Successful clinical trials will validate the approaches derived from Lee’s research, potentially changing the landscape of genetic disorder therapies. The emphasis on translating mechanistic understandings of chromosomal behavior into real-world interventions holds great promise. If successful, these trials can confirm not only the therapeutic efficacy but also the safety of treatments that leverage insights gained from decades of research into X chromosome inactivation, profoundly impacting patients’ lives.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in genetic disorders therapy?
X chromosome inactivation (XCI) is a biological process in which one of the two X chromosomes in female cells is turned off, preventing the overexpression of X-linked genes. This is crucial in genetic disorders therapy as many diseases, such as Fragile X Syndrome and Rett Syndrome, are caused by mutations on the X chromosome. By understanding XCI better, researchers like Jeannie T. Lee are developing methods to ‘unsilence’ mutated genes, paving the way for innovative therapies.
How does Jeannie T. Lee’s research contribute to understanding X chromosome inactivation?
Jeannie T. Lee’s research at Harvard focuses on unraveling the mechanisms behind X chromosome inactivation. Her lab discovered that a gelatinous substance surrounding chromosomes plays a key role in this process. By manipulating this chromosomal silencing, her team aims to develop treatments for X-linked genetic disorders like Fragile X and Rett Syndromes, potentially restoring gene function that is otherwise silenced.
What role does chromosomal silencing play in Fragile X Syndrome treatment?
Chromosomal silencing is a crucial aspect of X chromosome inactivation, and understanding it is vital for developing effective Fragile X Syndrome treatments. Mutations that cause this disorder are typically present on one X chromosome. By targeting the silenced X chromosome to reactivate healthy genes, researchers are exploring new therapeutic options that could significantly improve outcomes for individuals affected by Fragile X.
What are the implications of X chromosome inactivation for Rett Syndrome research?
In Rett Syndrome research, X chromosome inactivation has significant implications since the disorder is linked to mutations on the X chromosome. Research led by Jeannie T. Lee is investigating how unsilencing these mutations can restore gene function, offering hope for future therapies that could alleviate or reverse the symptoms of Rett Syndrome by leveraging our knowledge of chromosomal silencing.
How might recent findings on X chromosome inactivation benefit therapies for genetic disorders?
Recent findings on X chromosome inactivation, particularly those from Jeannie T. Lee’s lab, suggest that by understanding how to manipulate chromosomal silencing, we could create targeted therapies for genetic disorders like Fragile X Syndrome and Rett Syndrome. By potentially freeing inactivated X chromosomes, researchers may restore normal gene function without affecting other healthy genes, leading to safer and more effective treatments.
| Key Points | Details |
|---|---|
| What is X chromosome inactivation? | X chromosome inactivation is a biological process where one of the two X chromosomes in females is silenced to avoid excess gene dosage. This ensures that both males (with one X) and females (with two X’s) express similar amounts of X-linked genes. |
| Role of Jeannie Lee’s lab | Jeannie T. Lee’s lab at Mass General has significantly contributed to understanding how X chromosome inactivation occurs, focusing on the mechanisms that lead to chromosomal silencing. |
| Mechanism of Inactivation | X chromosome inactivation relies on a gelatinous substance (‘Jell-O’) that coats chromosomes. A gene called Xist interacts with this substance to change its properties, thereby silencing the X chromosome. |
| Potential Therapies | Research is being conducted to potentially cure genetic disorders like Fragile X Syndrome and Rett Syndrome by unsilencing genes on inactivated X chromosomes. |
| Clinical Trials | Future studies aim to optimize treatments derived from this research with plans to move towards clinical trials. |
| Remaining Mysteries | Although some genes regain function upon unsilencing, it is still unclear why healthy genes remain largely unaffected. Lee suggests limited cellular capacity to utilize genes may play a role. |
Summary
X chromosome inactivation is a crucial biological mechanism that balances gene dosage between males and females. This process, which has perplexed scientists for decades, reveals significant insights about how X-chromosome genes can be silenced in females while maintaining essential gene expressions. Through the groundbreaking work of Jeannie T. Lee and her lab, we are now closer to understanding this intricate process, which holds the potential for innovative therapies for serious genetic disorders, including Fragile X Syndrome and Rett Syndrome. As research advances and clinical trials approach, the possibility of restoring function to previously inactivated genes presents a promising future in genetic treatment.